Alzheimer’s disease is a neurodegenerative disease characterized by abnormal protein deposit aggregates in the brain tissue of patients. In particular, amyloid β-protein (Aβ) has been associated with the pathogenesis of Alzheimer’s. These Aβ molecules can aggregate and form oligomers, protofibrils, and mature fibrils.
Recent research suggests that Aβ protofibrils may be one of the most critical factors in the pathogenesis of the disease. However, antibodies targeting these structures are often not very specific and may cross among polymers, monomers, and oligomers. Therefore, researchers are keen to develop monoclonal antibodies targeting these specific structures to develop new therapeutic strategies.
Collaborated with Dr. Mickael Nichols’ team from the University of Missouri-St. Louis, Exonbio developed monoclonal antibodies that are highly selective for Aβ protofibrils using SPIN® technology.1
Immunization of rabbits
To develop such antibodies, we used purified Aβ42 protofibrils to immunize New Zealand White rabbits. Using rabbits for this purpose is beneficial as it generates more diverse antibodies with a higher affinity and greater stability than other animals often used for antibody production.
The rabbits were euthanized, and spleens and bone marrow were harvested for monoclonal antibody development. This process was conducted within a timeframe of two months.
Monoclonal antibody development using SPIN®
Subsequently, ExonBio’s unique Single Plasma Cell Interrogation (SPIN®) microfluidic technology was used to obtain individual antigen-specific plasma cells from rabbit spleenocytes. While most antibody development platforms use the overall B cell fraction or memory cells, we use plasma cells, a smaller, more mature population of cells. This maturity majorly increases the potential to find valuable antibodies and the number of antibody clones in a single run with one 96-well plate of cells.
SPIN® ensures the selection of highly specific antigen-specific plasma cells. Our methods allow for high throughput with an extremely high efficiency. SPIN® uses positive selection with the Aβ42 protofibrils and negative selection with monomers and polymers to select and obtain highly Aβ42 protofibrils specific plasma cells.

Figure 1: Overview of SPIN® workflow. Rabbits are immunized with biotinylated Aβ42 protofibrils, after which splenocytes are isolated and plasma cells are obtained using FACS. Antigen-specific plasma cells are isolated and obtained antibody sequences are cloned. Finally, ELISA screens are used to characterize the antibodies.
Obtained antibodies are highly selective
Candidate antibodies were assessed using ELISA screens, and selected antibodies were sequenced. When the researchers compared the sequences of some of the protofibril-selective antibodies with non-selective antibodies, they found consistent differences, even though there was a high similarity overall. This analysis revealed specific locations of interest potentially playing a role in the conformational selectivity for the Aβ protofibrils.
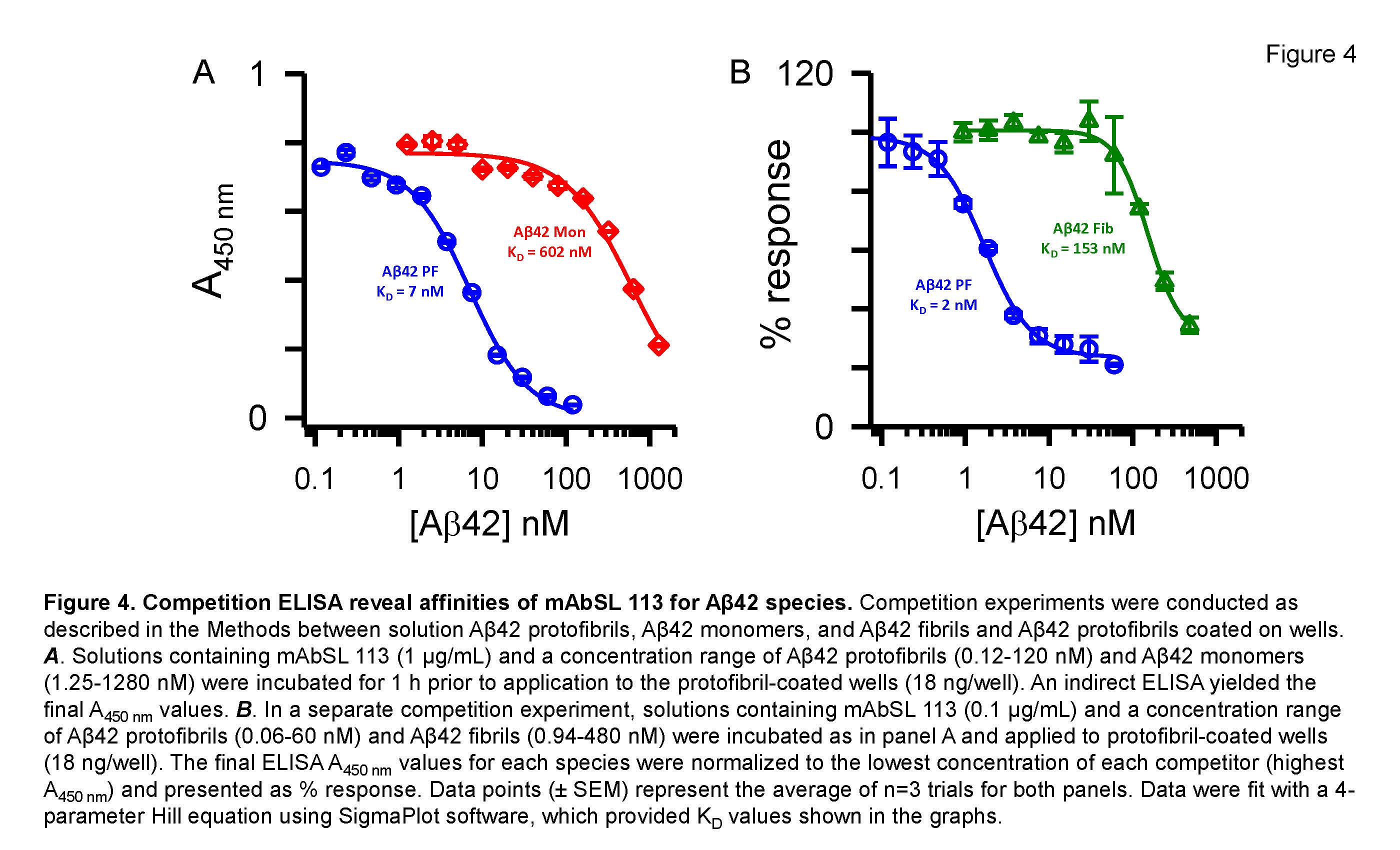
The figure shows that one of the selected purified antibodies (mAbSL 113) had high affinity and selectivity for Aβ42 protofibrils compared to Aβ42 monomers and fibrils.
“We developed and characterized a new class of monoclonal antibodies (referred to as mAbSL) that are selective for Aβ protofibrils over monomeric or fibrillar Aβ. The value of new antibodies with greater selectivity is manifold as
-
- Antibodies remain a major determinant of structural identification for aggregated species in AD brain tissue and fluid samples, and
- The ongoing (and completed) clinical trials assessing conformation-selective Aβ antibodies show some signs of clinical efficacy.”
Not only that, but in subsequent experiments, the researchers showed that this antibody significantly inhibited Aβ42 monomer aggregation by a unique mechanism and could impact Aβ dynamics.
“The pattern of inhibition by mAbSL 113 was suggestive of a mechanism in which soluble Aβ42 protofibrils were formed, rapidly bound by antibody, and their further assembly was suppressed.
In conclusion, from immunization to high-throughput SPIN procedure, to antibody clone devolution and expression, we produced a highly Aβ42 protofibrilspecific high affinity antibodies.
References:
- Grover, S., Pham, T., Jones, A., Sinobas-Pereira, C., Villoch Diaz Maurino, M., Garrad, E. C., Makoni, N. J., Parks, A., Domalewski, R. J., Riggio, G., An, H., Chen, K., & Nichols, M. R. (2023). A new class of monoclonal Aβ antibodies selectively targets and triggers deposition of Aβ protofibrils. Journal of Neurochemistry, 00, 1– 14. https://doi.org/10.1111/jnc.15817